NEUROMUSCULAR TOXINS:
BOTOX / DYSPORT / XEOMIN
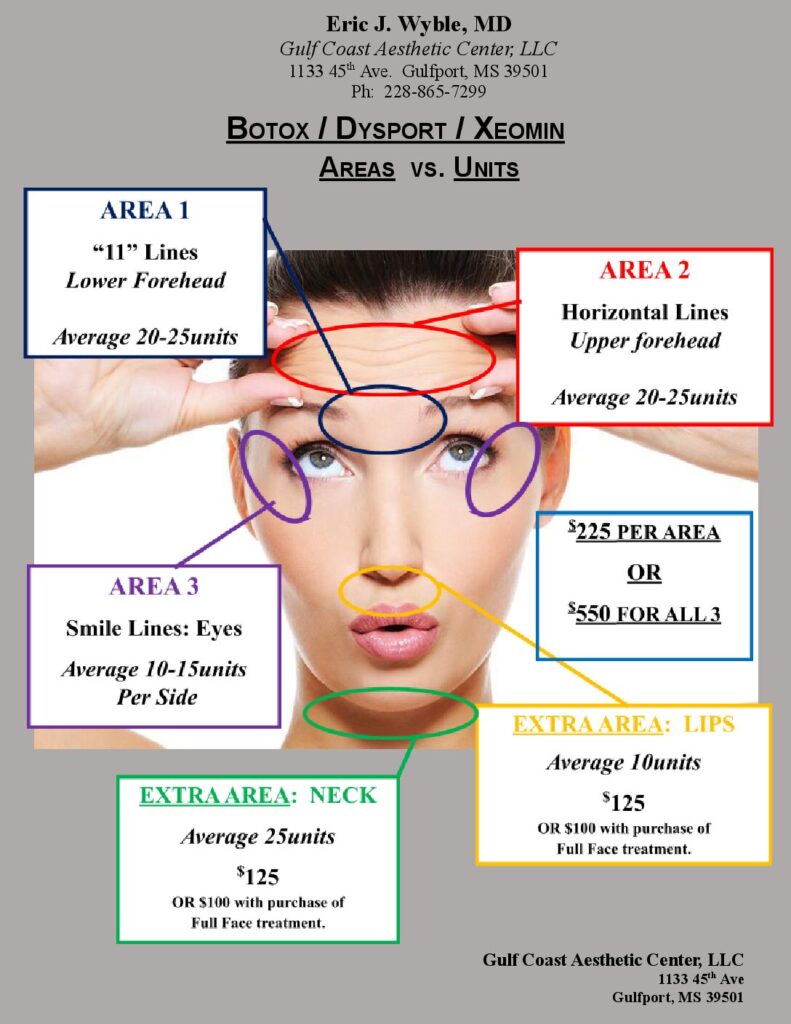
PRICE PER TREATMENT:
$225 per area, as seen in diagram.
$550 for all 3 areas (Dr Wyble’s “Full-Face” package).

Botox® Cosmetic
BOTOX® COSMETIC is indicated for the temporary improvement in the appearance of moderate to severe glabellar and lateral canthal lines associated with corrugator and/or procerus muscle activity in adult patients < 65 years of age. BOTOX® Cosmetic for injection, is sterile, vacuum-dried purified protein botulinum toxin type A, produced from fermentation of Hall strain Clostridium botulinum type A grown in a medium containing casein hydrolysate, glucose, and yeast extract, intended for the intramuscular use. BOTOX® Cosmetic blocks neuromuscular transmission by binding to acceptor sites on motor nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine. This inhibition occurs as the neurotoxin cleaves SNAP-25, a protein integral to the successful docking and release of acetylcholine from vesicles situated within nerve endings. When injected intramuscularly at therapeutic doses, BOTOX® Cosmetic procedures partial chemical denervation of the muscle resulting in a localized reduction in muscle activity.
Administration of BOTOX® Cosmetic is not recommended during pregnancy. There are no adequate and well controlled studies of BOTOX® Cosmetic in pregnant women. It is not known whether this rug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised with BOTOX® Cosmetic is administered in nursing women.
____________________________________________________________________________
Dysport ™
DYSPORT ™ is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients < 65 years of age. The effects of DYSPORT ™ and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effect. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults, in those patients who have underlying conditions that would predispose them to these symptoms.
DYSPORT ™ is contraindicated in patients with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation. This product may contain trace amounts of cow’s milk protein. Patients know to be allergic to cow’s milk protein should not be treated with DYSPORT ™. DYSPORT ™ is contraindicated for use in patients with infection the proposed injection site(s).
There are no adequate and well-controlled studies in pregnant women. DYSPORT ™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known where DYSPORT ™ is excreted in human milk.
_____________________________________________________________________________
Xeomin ®
XEOMIN® is an acetylcholine release inhibitor and neuromuscular blocking agent indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients. XEOMIN® is a prescription medicine that is injected into muscles and used to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults for a short period of time (temporary).
XEOMIN® may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems anytime (hours to weeks) after treatment with XEOMIN®:
Problems with swallowing, speaking, or breathing can happen within hours to weeks after an injection of XEOMIN® People with certain breathing problems may need to use muscles in their neck to help them breathe and may be at greater risk for serious breathing problems with XEOMIN®. Swallowing problems may last for several months, and during that time you may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving XEOMIN® have the highest risk of getting these problems. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include: loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing. These symptoms can happen hours to weeks after you receive an injection of XEOMIN®. These problems could make it unsafe for you to drive a car or do other dangerous activities.
Before receiving BOTOX, DYSPORT, and/or XEOMIN, tell your doctor about ALL of your medical conditions.
